Constitution of JPAC
1. Introduction
2. Accountability
3. Framework Organisational Chart
4. Remit and Terms of Reference of JPAC
5. Remits and Terms of Reference of SACs and WGs
6. Reporting Arrangements
7. Main Outputs from JPAC
8. Budget
9. Summary
1. Introduction
The structure and function of the Joint UKBTS Professional Advisory Committee (JPAC) and associated Standing Advisory Committees, originally created in 1987, have been subject to comprehensive review since 1998.
Changes in the organisation and management of the Transfusion Services throughout the UK, in 1997, resulted in the setting up of 4 National Services and the introduction of individual and statutory accountability vested in Chief Executives, for clinical governance and controls assurance. Devolution of governments has also empowered the individual Health Departments to make their own policy decisions.
The UK Forum, created in 1999, consisting of the Chief Executives and Medical Directors of the 4 Services, has taken a lead in these discussions and solid foundations and clear lines of accountability have been laid for the advisory committees which produce the professional guidelines for the Services.
In July 2000 the UK Forum agreed the terms of reference for the Red Book organisation:
- To prepare detailed service guidelines for the United Kingdom Blood Transfusion Services. These will constitute the professional advice to the Services. They should be reviewed regularly, at present annually.
- To be an advisory committee to the United Kingdom Transfusion Services, normally by reporting to the Medical Directors of the individual Services, who are themselves individually accountable to the Chief Executives.
Decisions on implementation and policy would be vested in the individual Chief Executives and their Service Boards and where appropriate their respective Health Departments.
To reflect this clarity of role the name of Joint UKNBTS/NIBSC Executive Liaison Committee was changed to Joint UKBTS/NIBSC Professional Advisory Committee on 14 June 2001. In 2013 the name was again changed to Joint UKBTS Professional Advisory Committee (JPAC) to reflect the inclusion of the MHRA and HTA, and that only the four UK Blood Services contribute financially to JPAC.
As in 1987, when the guidelines were first envisaged, they are produced to help ensure as far as possible and in the light of current knowledge the purity, potency, safety and efficacy of the donated blood in the UK without losing sight of availability.
Over the past years the guidelines have expanded to cover the safety of tissues and haemopoietic stem cells (1999) and the clinical use of blood and blood products (2000).
The guidelines aim to be evidence based as far as possible, although much of transfusion medicine is custom and practice based, and in many areas appropriate evidence by 21st Century standards cannot be obtained. Where this is the case, the lack of such evidence will be drawn to the attention of the reader.
The guidelines are published as the Red Book and support the good manufacturing standards against which the Regulatory Bodies inspect and licence the services.
The Services may deviate from these guidelines; however any such deviation will need to be substantiated and documented.
Guidelines for the clinical use of blood and blood products are published as the Handbook of Transfusion Medicine and will be reviewed at appropriate intervals.
The guidelines must take account of relevant Recommendations and Directives from the European Community.
2. Accountability
2.1. Lines of accountability
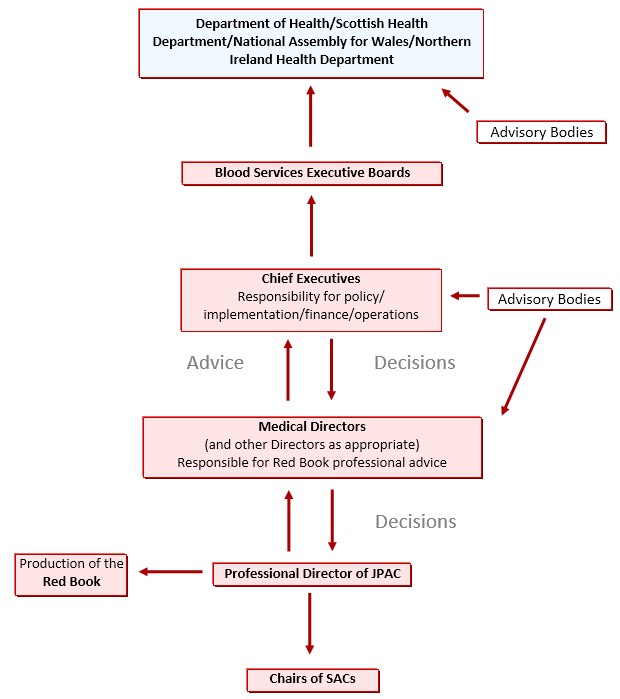
2.2. Confidentiality
The work of JPAC should be as transparent as possible. Unless there is a justifiable reason not to, papers on which recommendations from JPAC are based will be made publically available on the JPAC website after approval by JPAC. Reasons that this might not be possible include containing confidential information or that the paper may be published in a peer-reviewed journal. Confidential material might include, but is not limited to, data of commercial sensitivity. As a course of their duties members of JPAC and its SACs may view information that is confidential in nature. Papers that contain confidential information will be clearly marked as such, and members are expected to respect the confidentiality of such material and not discuss or distribute the material outside of JPAC or its SACs. In some instances it may be necessary to post JPAC papers on the web site prior to peer-review publication. This may be due to there being an urgent need to making data publically available. This should be discussed with the Professional Director of JPAC.The work of JPAC should be as transparent as possible. Unless there is a justifiable reason not to, papers on which recommendations from JPAC are based will be made publically available on the JPAC website after approval by JPAC. Reasons that this might not be possible include containing confidential information or that the paper may be published in a peer-reviewed journal. Confidential material might include, but is not limited to, data of commercial sensitivity. As a course of their duties members of JPAC and its SACs may view information that is confidential in nature. Papers that contain confidential information will be clearly marked as such, and members are expected to respect the confidentiality of such material and not discuss or distribute the material outside of JPAC or its SACs. In some instances it may be necessary to post JPAC papers on the web site prior to peer-review publication. This may be due to there being an urgent need to making data publically available. This should be discussed with the Professional Director of JPAC.
2.3. Indemnity
The work carried out by members of JPAC and SACs is considered to be part of their normal professional duties, which are carried out with the approval of their employing authority.
NHSBT is the legal and financial entity for JPAC governance and it has been confirmed that indemnity for members of JPAC is covered by the Liabilities to Third Parties Scheme under the NHSLA while undertaking activities for NHSBT.
2.4. Declarations of Interests
All members will need to declare any interests. SAC Chairs will ask for such declarations in writing annually, declaring any new interests at each meeting. These annual declarations will be kept by the JPAC office.
2.5. Continuing Professional Development (CPD) and appraisal
All members are expected to partake in CPD as deemed appropriate by their professional bodies.
All members working for JPAC are entitled to up to 10 CPD credits annually, depending on their CPD scheme. It is left to the individual professional to claim the number they deem appropriate.
All members must participate in an annual appraisal process with their employing authority. The Chair of JPAC will appraise SAC Chairs, and SAC Chairs their members, covering the scope of their work with JPAC and provide them with written feedback to contribute to this process.
3. Framework Organisation Chart
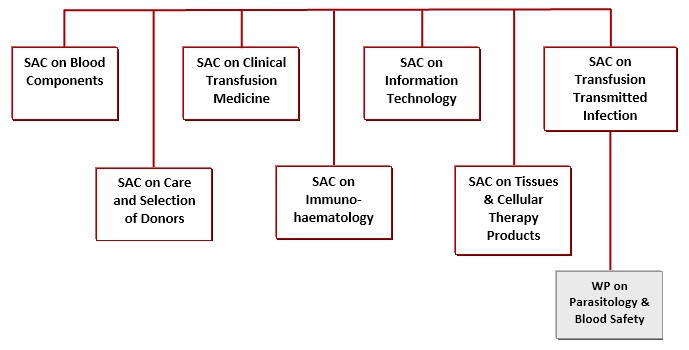
4. Remit and Terms of Reference of JPAC
The committee consists of the Professional Director of JPAC, Deputy Professional Director of JPAC, JPAC Manager, Scientific Lead Safety Policy (JPAC/SaBTO), chairs of the Standing Advisory Committees, Medical Directors of the 4 UK Blood Services, a UK Blood Service Quality Manager and representation from the Human Tissue Authority (HTA), the Irish Blood Transfusion Service (IBTS), the Medicines and Healthcare products Regulatory Agency (MHRA), the National Institute for Biological Standards and Control (NIBSC) and Public Health England (PHE)
Nominated deputies may attend meetings.
The Chief Executives of the Services may attend any meeting as observers or, if requested by JPAC, as participants.
4.1. The overall remit of JPAC
- Be responsible for the detailed guidelines for the UK Transfusion Services, produced as the Red Book.
- Be an Advisory Committee to the UK Transfusion Services. Since the knowledge base is expanding rapidly all the professionals involved must keep their own knowledge up to date so the advice is always timely and takes account of scientific and technological developments.
The guidelines for the UK also need to take account of the regulatory framework in this area, in particular that of the European Union and the UK Blood Safety and Quality Regulations.
Any expansion of JPAC has to be approved by the UK Forum.
It is the responsibility of JPAC to ensure that all relevant aspects dealing with the safety of blood and tissues in the UK are covered, and that the professional advice emanating from JPAC is communicated in a timely fashion to the UK Forum for further decision making if required.
JPAC is not concerned with implementation issues, nevertheless, as JPAC is responsible for producing the guidelines it is important that JPAC is kept informed of implementation arrangements and dates within the UK Services.
To deliver this remit JPAC needs to:
- oversee and co-ordinate the work of the SACs so as to develop, maintain, produce and communicate the guidelines
- discuss and endorse the guidance emanating from the SACs
- ensure that all aspects relevant to the safety of blood and tissues are covered by the various committees
- ensure that appropriate discussions are held at the SACs and that the guidelines are based on evidence wherever possible and consistent with the legislative environment
- approve proposed sub-committees and working parties and their remits
- ensure the timely updating of the guidelines so they always reflect current best practice in the UK
- suggest areas for further exploration to appropriate bodies
- maintain close collaboration with NIBSC
- support the Professional Director in the preparation of reports and annual work plans for the UK Forum.
4.2. Meetings
There will be at least 3 meetings annually.
The agenda items are submitted by members of JPAC and the UK Forum.
If a member of JPAC is unable to attend they may appoint a deputy who should be fully briefed.
If fewer than 2 Medical Directors are able to attend a particular JPAC meeting, then approval of any decisions made by the committee members present will be sought from the Medical Directors following the meeting and prior to distribution of the draft minutes.
4.3. Minutes
The secretary to the JPAC meeting will take minutes and circulate the draft version to committee members for any corrections. The minutes will then be formally approved at the next meeting and then posted on the JPAC website.
4.4. Roles and Responsibilities of JPAC
4.4.1. Professional Director of JPAC
4.4.1.1. Appointment Procedure
Vacancy of the Professional Director position will be advertised through the Medical Directors, and if deemed appropriate by the UK Forum, by external advertisement.
The post is funded through the JPAC budget and is 0.6 WTE
Application will be by Curriculum Vitae and a covering letter, outlining the reason for application and key attributes which make the candidate suitable for the post.
Appointment will be made by the Medical Director of NHSBT in conjunction with the UK Forum and will include an interview process determined by the UK Forum.
To avoid any conflict of interest the Medical Directors, Chief Executives or individuals who hold national functional lead roles for implementation within their respective services, will not be eligible to stand as Professional Director.
4.4.1.2. Responsibilities of Professional Director of JPAC
- Provide professional direction to JPAC and its SACs
- Organise meetings of JPAC
- Set agenda based on submitted issues and ensure that papers for the meetings are sent out at least one week prior to a meeting
- Co-ordinate the work of all SACs; this may involve attending meetings of the SAC and in case of absence of a chair or deputy chairing the meeting
- Approve proposed SAC membership nominations by SAC chairs
- Together with JPAC approve membership, remit and work plans of any working parties
- Hold annual review meetings with each of the SAC Chairs to review the SAC membership, terms of reference and work plan of each individual committee and discuss any issues
- Appraise the work of SAC Chairs annually and provide written feedback to them for inclusion in their appraisal process with their employing authority
- Prepare an annual work plan for the work of JPAC and its SACs for submission to the UK Forum
- Organise appointments of the chairs of SACs
- Ensure timely updates of the Red Book and work with the publishers
- Arrange distribution according to agreed procedures
- Ensure that all printed and electronic versions are up to date
- Ensure that www.transfusionguidelines.org.uk (launched 2002) is maintained according to the wishes of JPAC and the users
- Maintain close links with the HTA, MHRA, NIBSC, PHE and SaBTO
- It is expected that the Professional Director of JPAC is the UK representative on the Council of Europe (CoE) CD-P-TS and GTS Committees and attends the meetings. This ensures appropriate co-ordination of CoE and UK guidelines
- It is also expected that the Professional Director of JPAC is kept informed of and plays a part in developments in the European Union (EU) in respect of transfusion medicine
- Communicate with all SAC chairs to keep them up to date with CoE guidelines and developments in EU
- Ensure that a functional archive of JPAC work is maintained together with an audit trail of decisions made
- Promote the Red Book as guidelines to the Services, and also promote the Handbook of Transfusion Medicine as guidelines for the Hospitals
- Keep within the allocated budget
4.4.2. Responsibilities of Deputy Professional Director of JPAC
- To deputise for the Professional Director of JPAC when required due to annual leave, professional or sickness absence:
- Chair JPAC meeting
- In conjunction with the JPAC Manager, respond to urgent requests for information from JPAC, UK Forum, Blood Services or other healthcare professionals
- Assess urgent new potential transfusion risks and coordinate response to them
- To support the Professional Director in developing and delivering the JPAC Work Plan through regular meetings and consultation
- To attend JPAC meetings and JPAC EWG teleconferences
The Deputy Director should have experience of work for JPAC, or one of its SACs, and current or previous experience of Chairing an SAC is desirable.
The post is not remunerated by JPAC, but time to undertake the role should be negotiated with his / her employer and recognised in their job plan.
4.4.3. Responsibilities of JPAC Manager
- To provide support to the Professional Director of JPAC and the Standing Advisory Committee (SAC) Chairs, assisting, developing and maintaining the partnership between the Joint UKBTS Professional Advisory Committee (JPAC), its stakeholders and the healthcare community in the United Kingdom
- Project manage the production of JPAC publications including the Guidelines for the Blood Transfusion Services in the United Kingdom and the Handbook of Transfusion Medicine
- Play a lead role in promoting the objectives of JPAC and work with the Professional Director of JPAC and the SAC Chairs to ensure that JPAC led areas of work are implemented
- Manage the JPAC website www.transfusionguidelines.org.uk and act as the main link to the website service providers
- Be the budget holder and authorised signatory for the JPAC budget
- Responsible for the line management, training and development of the post of JPAC Administrator
- Responsible for developing robust management of all areas of JPAC work including the JPAC website and archive, ensuring that a consistent and professional level of service is achieved
- Contribute to the strategic direction of JPAC
- Develop key relationships with the 4 UK Blood Services
- Act as an outward-facing link for JPAC and the JPAC website
4.4.4. Responsibilities of Scientific Lead Safety Policy (JPAC/SaBTO)
- To work within the secretariat of the DH Advisory Committee for the Safety of Blood, Tissues and Organs (SaBTO) to support members of SaBTO and its sub-groups by producing policy papers, reports and summaries and in planning of work plans and meeting agendas
- To provide scientific leadership in production of policy papers to the UK Blood Services Joint Professional Advisory Committee (JPAC) and its Standing Advisory Committees, working closely with the Chair and Deputy Chair of JPAC, Chairs of SACs and JPAC Manager
- To provide co-ordination of work relating to the safety of Blood, Tissues and Organs by providing liaison between different decision-making bodies
- To be the scientific secretary for the UK Blood Services Prion Working Group and related activities
- To develop in depth familiarity with safety frameworks used by JPAC and SaBTO, and to work with project teams on their use
- To produce reports for Blood Service Boards and DH as required
5. Remits and Terms of Reference of Standing Advisory Committees, Sub-Committees and Working Groups
The remits of the SACs delineate the fields of transfusion medicine and tissue banking covered by the particular SAC, the terms of reference detail the workings of a particular SAC.
Standing advisory committees and sub-committees are established and disbanded according to the needs of the UK Services following JPAC and UK Forum approval.
Working groups are “ad hoc” groups set up by SACs, with JPAC approval, to consider a specific issue within a clearly defined time span and report back to the parent SAC; following completion of the task they will be disbanded.
Although the remits of the SACs vary, the general broad terms of reference apply to all. All SACs have written terms of reference which detail their manner of working: meetings, minutes, distribution of minutes, membership.
All SACs report to JPAC through their Chairs.
All SACs are under an obligation to maintain the appropriate section of the Red Book to ensure that the guidance is up to date and based, wherever possible, on evidence. If the evidence on which guidelines should be based is not available, as may be the case with time honoured custom and practice, the SAC should suggest to JPAC the best way of obtaining such evidence. Where it is clear that evidence for practice does not exist, and cannot be obtained, this must be clearly acknowledged.
All SACs have to take account of the regulatory framework (UK and European) for their field of practice.
All SACs are under an obligation to comment, when requested, on consultation documents relevant to their field of practice.
5.1. Chairs of SACs
The Chairs and SAC members serve in a personal capacity due to their own expertise and not as representatives of any group, although account has to be taken of the needs of the 4 Services in the UK.
The chairs are appointed for by the Medical Directors and the Professional Director of JPAC. They are accountable to the Professional Director of JPAC, and their appointment will be reviewed every 3 years.
During the appointment process efforts should be made to ensure continuity of the work of a SAC.
5.1.1. Appointment Procedure
Vacancy of a Chair position will be advertised through the Medical Directors and expressions of interest sought from potential candidates within JPAC and the 4 UK Blood Services.
Application will be by Curriculum Vitae and a covering letter, outlining the reason for application and key attributes which make the candidate suitable for the post.
Appointment will be made by the Professional Director of JPAC in conjunction with members of the UK Forum, and will include an interview process.
To avoid any conflict of interest the Medical Directors and Chief Executives of the Services will not be eligible to stand as Chairs of SACs, they are however eligible as members of SACs.
Individuals who hold national functional lead roles for implementation within their respective services should not normally hold chairs of SACs relevant to their main functional area of responsibility, but may serve as members.
5.1.2. Responsibilities of Chairs of SACs
Appoint members according to the rules as outlined in the JPAC constitution.
Perform annual reviews with members as part of their appraisal or performance review.
Set agendas for meetings; ensure that relevant topics are discussed.
Ensure that horizon scanning in the relevant area is a standing item on every SAC meeting agenda.
Ensure that SAC recommendations are presented to JPAC and endorsed in a timely manner prior to incorporation in guidelines.
Ensure timely update of relevant chapters and supplements.
Ensure that the time of the experts is used most effectively. (E.g. using audio conferencing facilities, asking a small group from the SAC to undertake the practical updating of the guidelines).
Ensure that relevant European Documents are read and commented on as requested. This task may need to be delegated to a few members, which should include the Chair.
Ensure that appropriate communication takes place with other relevant groups within the 4 UK Blood Services.
5.2. Members of SACs
Members of SACs will be appointed by the SAC Chair in consultation with their SAC and the Professional Director of JPAC. The number of members will be left to the discretion of the Chair, but generally no more than 10 would be expected. Overlap of membership should be ensured by the Chair to avoid turnover of the whole committee at the same time.
Although members are appointed for their personal expertise the Chair should ensure that there are members from at least 2 of the Services on their committee to bring wider experience to the SAC. It is advisable that there be some cross representation between SACs.
It is also advisable that there are members from outside the UK Blood Transfusion Services to give the SACs more authority throughout transfusion medicine. For some SACs lay representation is encouraged.
In view of the complexity of the guidelines and increased workloads maximum flexibility should be allowed and all SACs need to work efficiently and effectively.
Appropriate succession planning and encouragement of younger members to serve on the Advisory Committees is encouraged.
Observers can attend SAC meetings at the discretion of the SAC Chair.
5.3. Remits of SACs
The remits of the SAC are reviewed annually.
General to all Standing Advisory Committees
- Prepare detailed service guidelines for the UK Blood Transfusion Services, taking account of the Blood Safety and Quality Regulations (2005), the Human Tissue (Quality and Safety for Human Application) Regulations 2007 and future UK legislation affecting the blood and tissue services
- Provide professional and technical advice (where appropriate) to the four UK Blood Services
- Provide documented summaries of the evidence and arguments on which these service guidelines are based
- Keep these guidelines up to date in the light of developments in scientific and medical knowledge and changes in the regulatory environment, consistent with good practice and the “state of the art” provisions of the EU Directives and UK Regulations
- Provide scientific, medical, technical (where appropriate) information for staff training
- The Chair, as a member of JPAC has a special responsibility for effective working with the other SACs
- Ensure JPAC is kept informed of any significant developments both nationally and internationally within the remit of the SAC
5.3.1. Standing Advisory Committee on Blood Components (SACBC)
- Set specifications for blood components, evidence-based where possible
- Develop and review validation of novel blood components
- Assess acceptability for use of novel blood components
- Assess and set requirements for storage and transport systems for blood components
- Co-ordinate with the Standing Advisory Committee on Information Technology regarding labelling and unique identification of blood components and maintenance of the product portfolio (website)
- Develop generic protocols for evaluating methods for the collection and processing of blood and blood components
- Contribute content to the JPAC website (background papers and references etc.)
- Co-ordinate with other SACs and, other relevant UK Working Groups, as appropriate
5.3.2. Standing Advisory Committee on Care and Selection of Donors (SACCSD)
- Set, and update as required, guidelines for:
- Care, pre and post donation, of people who offer to donate blood and components
- Donor selection to identify and exclude those for whom the act of donation could be unsafe
- Donor selection to identify and exclude those whose donation could be unsafe, of inadequate quality, or contrary to relevant legislation
- Staffing, environment, equipment and procedures for blood donation sessions
- Liaise with the Standing Advisory Committee on Tissues and Cellular Therapy Products regarding donor selection criteria
- Co-ordinate with the Standing Advisory Committee on Transfusion Transmitted Infection to ensure integrated advice on all aspects of microbiological safety of donors and donations
5.3.3. Standing Advisory Committee on Clinical Transfusion Medicine (SACCTM)
- Provide JPAC with advice, updates and relevant information from the clinical user community
- Provide a link from JPAC to clinical users through other organisations e.g. BCSH, National Blood Transfusion Committee etc.
- Provide a clinical users’ reference point for the other JPAC SACs
- Ensure the Handbook of Transfusion Medicine (HTM) remains up to date and clinically relevant. The HTM to include information on:
- Blood component manufacture and composition for clinical users
- Clinical indications and contraindications
- Prescription ordering and administration
- Observations, adverse events and reactions, management
- Documentation and traceability
- Patient information and consent
- Blood conservation and alternatives
- Respond to requests for clinical information/advice
5.3.4. Standing Advisory Committee on Immunohaematology (SACIH)
- Set guidelines on immunohaematology testing of donors and patients by serological and NAT methodology (red cells, white cells and platelets)
- Liaise with other SACs, especially SAC-Blood Components and SAC for Care and Selection of Donors, in areas where there is an Immunohaematology interest (e.g. HLA screening assays)
- Liaise with NIBSC on availability, development and use of standard reference preparations and reagents for Immunohaematology, Histocompatibility and Immunogenetics (e.g. for red cell, platelet, anti-HLA, anti-HNA serology and for antigen genotyping.)
5.3.5. Standing Advisory Committee on Information Technology (SACIT)
- Define standards and/or guidance for inclusion within the “Guidelines for the Blood Transfusion Services in the UK” (the Red Book), sometimes in conjunction with other standing advisory committees. These standards and/or guidance will cover all services provided by the UKBTS
- Review relevant international and NHS guidelines, legislation and developments in IT and assess their relevance for the UK
- Promote commonality and define and recommend standards for the UK services to JPAC. This will include standards on data structures, delivery mechanisms and labelling
- Administer the UK services’ database on blood component labels and barcodes
- Recommend, support and advise on (but not to implement) strategies and change programmes for the implementation of standards in the UK services and hospitals
- Promote the use of electronic communication and data interchange
- Maintain relationships with national and international groups to understand better the development of standards in IT. This will include Tissue Services standards groups and Stem Cell standards groups
5.3.6. Standing Advisory Committee on Tissues and Cellular Therapy Products (SACTCTP)
- Provide high quality professional advice on matters relating to tissue and cellular therapy products. This will include
- the assessment, counselling, consent and care of donors or relatives
- the collection of tissues and cells and their processing, quality assessment, storage, transportation and issue for clinical use or other purposes
- the labelling and unique identification of tissue and cellular products
- adverse event reporting
- Be responsible for recommending the content of, and any changes to, the chapters related to tissues and cellular therapy products in the Guidelines for UKBTS (Red Book)
- Be responsible for the content and any changes to the UKBTS Donor Selection Guidelines for
- living donors of tissue
- deceased donors of tissue
- bone marrow and peripheral blood stem cells
- cord blood
- Co-ordinate with the Standing Advisory Committees on Care and Selection of Donors, Transfusion Transmitted Infection and Information Technology as required
5.3.7. Standing Advisory Committee on Transfusion Transmitted Infections (SACTTI)
- Maintain awareness of new or previously unrecognised (“emerging”) microbiological threats to safety of blood and tissues
- Advise on the epidemiological basis for targeting or avoiding particular groups as potential donors - in respect of both recognised and emerging transfusion transmissible agents
- Recommend laboratory and related procedures for detection and exclusion of donations that may pose a microbiological risk
- Co-ordinate with the Standing Advisory Committee on Care and Selection of donors and where appropriate prepare joint recommendations to JPAC that take account of all relevant aspects of microbiological safety of donors and donations
- Co-ordinate with the Standing Advisory Committee on Blood Components and the SAC on Tissues and Cellular Therapy Products on guidance to improve microbiological safety of donations
6. Reporting Arrangements
All chairs of the SACs will report on the progress of their previous year’s activities at their annual SAC review meeting. These will form part of the formal reporting process of the Professional Director of JPAC to the Medical Directors and the UK Forum.
All SACs and the Professional Director of the JPAC will produce work plans annually, indicating the topics they hope to cover during the year. The collated JPAC work plan is submitted to the UK Forum annually.
New developments during the year may arise and take precedence on the agendas of all groups.
Due to the complexity of the organisation it is clearly important that all the work carried out by the different groups is documented, considered and appropriate recommendations forwarded. The parent SAC is responsible for considering the work of any sub-committees and working parties and, where appropriate, bring issues to JPAC.
- The parent SAC must ensure that the work remains focused
- All sub-committees report to the parent SAC/SACs
- All working parties report to the parent SAC/SACs
- All SACs report to JPAC
7. Main Outputs from JPAC
7.1. Professional Advice
Considered, timely, and documented professional advice to the Medical Directors, and UK Forum on issues relevant to transfusion medicine and the safety of blood transfusion and tissue transplantation in the UK.
7.2. Guidelines for the Blood Transfusion Services in the United Kingdom (Red Book) - which presents the current professional guidelines
The content of the Red Book is reviewed regularly. Systems are in place to ensure that new guidelines, appearing in between editions of the Red Book, are communicated to the Services and appear formally in ensuing editions.
7.3. Handbook of Transfusion Medicine
The guidelines for the clinical use of blood, consistent with advice emanating from other professional organisations. The published book is the fundamental document but a version also appears on the website. This version is constantly improved and contains further explanations.
7.4. Whole Blood and Components Donor Selection Guidelines, Tissues and Cells Donor Selection Guidelines and the Geographical Disease Risk Index
These guidelines are issued separately as they change frequently. Systems are in place for controlling and documenting changes.
7.5. JPAC Website
The website, www.transfusionguidelines.org.uk, is maintained by the JPAC Manager in conjunction with the software providers.
7.6. JPAC Policy on the Publication of Work
JPAC actively encourages members of its SACs to publish work that has been conducted on behalf of JPAC, where relevant. This will facilitate:
- Independent peer-review
- Professional development of SAC members
- Raising the profile of JPAC and its SAC
7.6.1. Process
7.6.1.1. Assignment of authorship
The lead author of the article identifies all others that have made a contribution to the work and decides whether they meet ALL of the following criteria to be a co-author:
- they have made a significant contribution to the concept and design, acquisition of data/analysis and interpretation of data (for original articles)
- they have contributed to drafting or critical appraisal of the article
- they have approved the final version to be published
The lead author is responsible for ensuring that all co-authors have seen and approved the final article.
If there are too many authors to list, then consideration should be given to the lead author publishing the article on behalf of the UK Joint Professional Advisory Committee and listing contributors as collaborators.
7.6.2. Review by JPAC and copyright
Most journals ask authors to assign copyright to the publishers. To avoid issues with copyright, substantial elements of the publication should therefore not be reproduced on the JPAC website. Therefore, the usual sequence of events for material that is suitable for publication should be as follows:
- JPAC approve the paper on which the publication will be based
- If there needs to be modification of the JPAC paper to make it suitable for publication, then the Professional Director of JPAC must approve the submitted version
- Authors submit for peer-review and revise in light of comments
- Authors bring the paper back to JPAC if the peer-review process has raised any significant concerns
- Following peer review a link to the published paper is posted on the JPAC website. If the article does not contain a summary of the opinion given by JPAC then consideration should be given to posting a short summary of this along with the link to the publication
In some instances it may be necessary to post JPAC papers prior to peer-review publication. This may be due to there being an urgent need to making data publically available. This should be discussed with the Professional Director of JPAC.
8. Budget
A budget has been identified for JPAC. The 4 UK Blood Services contribute to this budget pro rata, as follows (March 2019):
- 84.2% - NHS Blood and Transplant, England (NHSBT)
- 8.2% - Scottish National Blood Transfusion Service (SNBTS)
- 4.7% - Welsh Blood Service (WBS)
- 2.8% - Northern Ireland Blood Transfusion Service (NIBTS)
The budget also provides for the hosting and maintenance of the website www.transfusionguidelines.org.uk.
Should an increase in the budget be required the Professional Director of JPAC will approach the UK Forum.
9. Summary
This document aims to outline the agreed constitution, lines of accountability and working methods for the professional advisory machinery responsible for producing the Red Book and supplements (all the Donor Selection Guidelines, including the Geographical Disease Risk Index) and the Handbook of Transfusion Medicine.
The rapid advance of knowledge in transfusion medicine makes it inevitable that the various advisory committees, subcommittees and working parties are flexible and so they can respond to new challenges. The basic mechanisms for such change are outlined in this constitution.
Updated March 2020