9.2: Microbiology screening
Note: The meanings of certain terms used in this section are defined in section 9.2.6.
9.2.1: Screening of donations/donors
Donation/donor screening can be broadly divided into two main categories:
- Mandatory: Absolute requirement prior to the release of components. There are, however, different reasons for a specific infectious marker to be defined as ‘mandatory’. These include a UK or European Union regulatory requirement, a specific instruction from the Department of Health, including its Advisory Committees, and an Act of Parliament.
- Additional (also known as Discretionary): Performed because of specific additional and identifiable donor or recipient risk/regulatory requirement.
Importantly, the mandatory requirements for blood donation and for tissue and stem cell donations are different, with some tests that are defined as ‘Additional’ for blood donations being ‘Mandatory’ for non-blood donations (Tables 9.1 and 9.2). Although not required for all donations, where additional screening is required, the results are an integral part of the criteria for the release of that donation/component/product. In addition, for certain donation types, there is the option of quarantine and follow-up serological screening before issue or the inclusion of genomic screening at donation.
Donations and any associated components/products must not be released to stock unless they have been screened and found negative for the mandatory, and any additional, microbiological screening required. In certain circumstances, for certain donation/component types, a reactive screen result may not preclude release of the donations/component.
Table 9.1 Screening required for blood donations
| Infectious agent | Minimum requirement | Comments1 |
|---|---|---|
| HIV 1+2 | anti-HIV 1+2+O or HIV 1+2+O Ag/Ab (M) HIV RNA2 |
RNA screening in pools of a maximum of 24 donations3 |
| HCV | anti-HCV (M) HCV RNA (M) |
RNA screening in pools of a maximum of 24 donations3 |
| HBV | HBsAg (M) HBV DNA2 anti-HBc [+ anti-HBs] (A)4 |
DNA screening in pools of a maximum of 24 donations3 Donations that are anti-HBc reactive and have anti-HBs >100 mIU/mL, tested in the past 24 months by a UK Blood Service, are considered suitable for release if HBsAg and ID HBV DNA negative |
| Syphilis | anti-treponemal (M) | |
| HTLV I/II | anti-HTLV I/II (M)5 | Serology screening individually or in pools of a maximum of 24 donations3 |
| HEV | HEV RNA (M) | RNA screening in pools of a maximum of 24 donations3 |
| HCMV | anti-HCMV (A) | Ideally both IgG and IgM, but IgG alone is considered sufficient |
| Plasmodium sp. | anti-P. falciparum/vivax (A) | |
| Trypanosoma cruzi | anti-T. cruzi (A) | |
| West Nile Virus (WNV) | WNV RNA (A) | RNA screening in pools of a maximum of 16 donations6 |
| HAV | HAV RNA (A) | RNA screening in pools of a maximum of 96 donations |
| Human B19 | B19 DNA (A) | DNA screening in pools of a maximum of 96 donations |
| (M) – mandatory (release criteria) for the purpose of these guidelines | ||
| (A) – additional (release criteria) due to specifically identifiable risk | ||
| 1 All microbiology screening performed on individual donations unless specified otherwise | ||
| 2 Although neither are mandatory for blood donations in most of the UK, HIV RNA and HBV DNA are included in nucleic acid screening as the commercial systems available are now triplex assays. HIV RNA is, however, mandated within Scotland. | ||
| 3 The minimum sensitivity of the molecular screening is dependent upon pool size. The maximum validated pool size for use for mandatory blood screening within the UK Blood Transfusion Services is 24 donations. | ||
| 4 All blood donors are to be screened for anti-HBc at their first donation or their first donation after the introduction of anti-HBc screening. Anti-HBc screening to be repeated if a donor lapses (over 2 years) or has a new HBV risk. | ||
| 5 anti-HTLV screening is only required for blood donations from previously untested donors and for blood donations destined for use to prepare non-leucodepleted products | ||
| 6 The maximum validated pool size for WNV RNA screening is 16 donations | ||
Table 9.2 Screening required for tissue and stem cell donations1
| Infectious agent | Minimum requirement | Comments1,2 |
|---|---|---|
| HIV 1+2 | anti-HIV 1+2+O or HIV 1+2+O Ag/Ab (M) HIV RNA (O) |
Stem cell donors: as for blood donors |
| HCV | anti-HCV (M) HCV Ag and/or HCV Ag/Ab (O) HCV RNA (O) |
Stem cell donors: as for blood donors |
| HBV | HBsAg (M) anti-HBc (M) [+ anti-HBs2] (O) HBV DNA3 (O) |
Stem cell donors: as for blood donors (for HBsAg and HBV DNA, anti-HBc mandatory for stem cell donors)
Either: donations that are anti-HBc reactive and have anti-HBs ≥100 mIU/mL are considered suitable for release Or: donations that are anti-HBc reactive and are HBsAg and ID HBV DNA negative do not require an anti-HBs level of ≥100 mIU/mL to be considered suitable for release3 |
| Syphilis | anti-treponemal (M) | |
| HTLV I/II | anti-HTLV I/II (M)4,5 | Serology screening individually or in pools of a maximum of 24 donations4 |
| HEV | HEV RNA (A) | RNA screening in pools of a maximum of 24 donations4 |
| HCMV | anti-HCMV (A) | Ideally both IgG and IgM, but IgG alone is considered sufficient |
| Plasmodium sp. | anti-P. falciparum/vivax (A) Plasmodium spp DNA (A)7 |
|
| Trypanosoma cruzi | anti-T. cruzi (A) | |
| West Nile Virus (WNV) | WNV RNA (A) | Maximum of 16 donations6 |
| (M) – mandatory (release criteria) for the purpose of these guidelines | ||
| (A) – additional (release criteria) due to specifically identifiable risk | ||
| (O) – optional, genomic screening for HIV, HCV and HBV nucleic acids is not mandated but can be performed on the original donation sample as an alternative to 180 days’ quarantine and follow-up serological testing | ||
| 1 All microbiology screening performed on individual donations unless specified otherwise | ||
| 2 UK screening requirements. Other testing, e.g. Epstein-Barr virus, toxoplasmosis, may be required as additional tests depending upon specific additional risk and/or special requests for individual recipients. For certain product types that are exported there may be additional end user screening requirements. | ||
| 3 anti-HBc reactive tissue and stem cell donations do not need to have an anti-HBs level ≥100 mIU/ml to be considered suitable for release if both HBsAg and ID HBV NAT are negative on screening | ||
| 4 All screening of deceased tissue donations should be performed on individual samples. Anti-HTLV I/II screening of surgical tissues/stem cells may be performed using pools of a maximum of 24 samples. HEV RNA screening of surgical tissues may be performed using pools of a maximum of 24 samples | ||
| 5 Not mandatory for avascular tissue donations but may be considered good practice | ||
| 6 The maximum validated pool size for WNV RNA screening is 16 donations. | ||
| 7 Certain tissues and cord bloods from donors with malaria risk and which are found to be malaria antibody positive may be released for use if additional testing for malaria DNA is performed and malaria DNA is not detected (see Tissue Donor Selection Guidelines3) | ||
9.2.2: Deceased neonatal and infant tissue donors
- Full microbiology screening of a maternal sample is always required.
- For still births and for neonates up to 28 days after birth, no microbiology screening of the neonate is required.
- For infants more than 28 days after birth, full microbiology screening of an infant’s sample is required.
9.2.3: Serology screening algorithms
9.2.3.1: Blood donations
- No donation which is initially reactive for the first time in the routine screening assay can be released for clinical use unless subsequently shown to have a negative result in both tests in duplicate repeat testing using the same assay.
- Blood donations which are reactive in one or both of the repeat tests are unsuitable for use and must be labelled as biological hazard/not for transfusion.
- Donations which are initially reactive in the routine screening assay, but which originate from donors who have been previously investigated in a reference laboratory and have been shown to be demonstrating non-specific reactivity, may be screened using a second (alternative) screening assay of at least equal sensitivity to the primary screening assay, and can be considered suitable for clinical use if giving a negative result in the alternative screening assay.
See flowchart for screening of blood donations, provided as Figure 9.1.
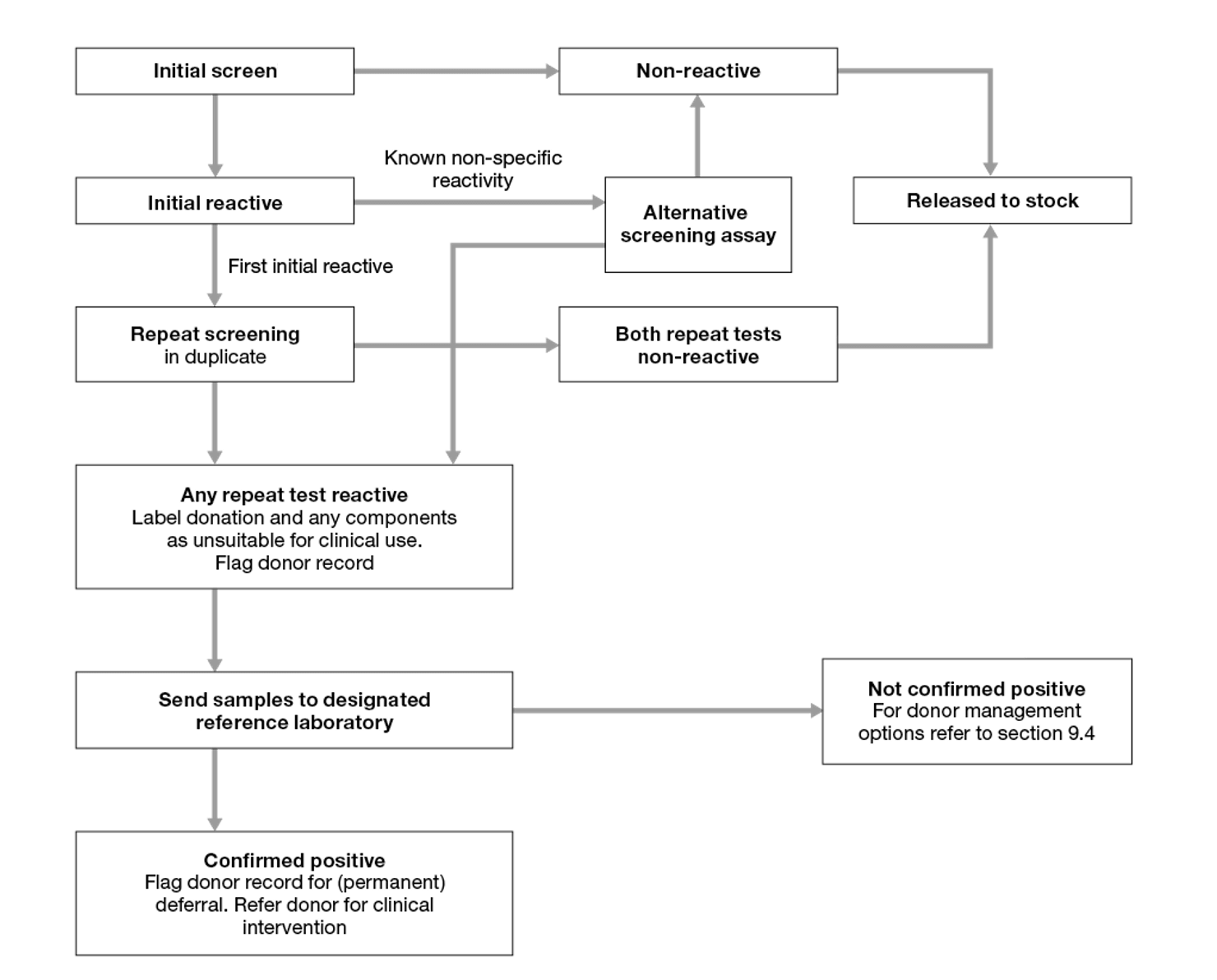
Figure 9.1 Serology screening: blood donations
9.2.3.2: Tissue and stem cell donations
- All initially reactive samples (see Figure 9.2) must be re-tested in duplicate using either the same assay or using an alternative assay that has been specifically evaluated to have at least equal sensitivity and ideally is based on different antigens and/or antibodies, and/or principles.
- Donations that are non-reactive in both of the repeat tests can be considered suitable for clinical use.
- Donations that are reactive in one or both of the repeat tests may in some clinical circumstances, and depending on the confirmatory results, be considered suitable for use (SaBTO Microbiological Safety Guidelines, 20203).
9.2.4: Molecular screening algorithm
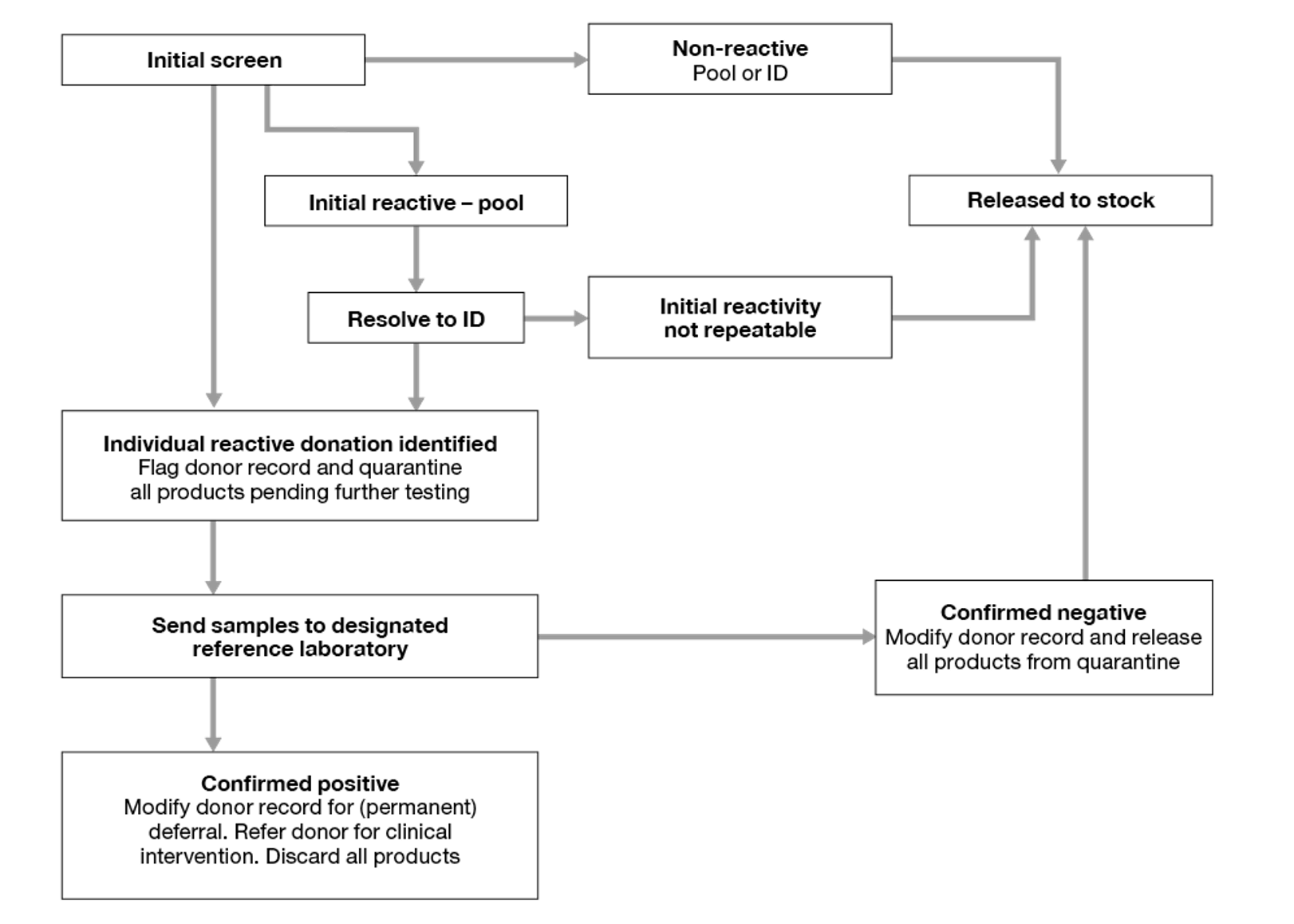
- All initially reactive pools (see Figures 9.3 and 9.4) must be resolved to an individual (or more) reactive donation(s). All other non-reactive donations can be considered suitable for clinical use.
- Individual reactive donations are unsuitable for clinical use and must be labelled as biological hazard/not for transfusion.
- Stem cell donations from known infected individuals that are reactive on screening may in some clinical circumstances be considered suitable for use (SaBTO Microbiological Safety Guidelines, 20203).
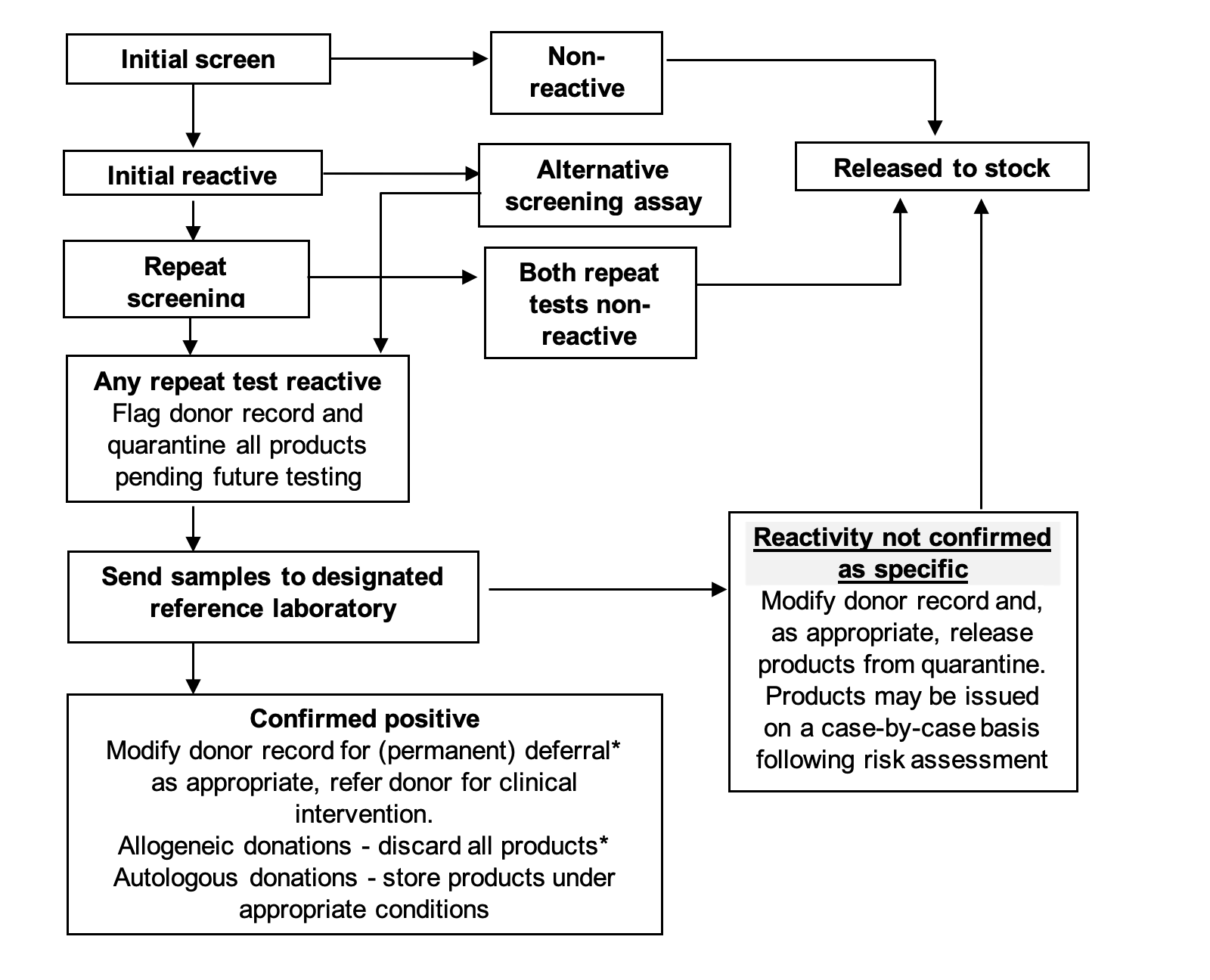
Figure 9.2 Serology screening: tissue and stem cell donors/donations
* Tissue/stem cell donors/donations confirmed to be anti-HBc reactive, and which are HBsAg and ID HBV DNA negative may be considered suitable for release
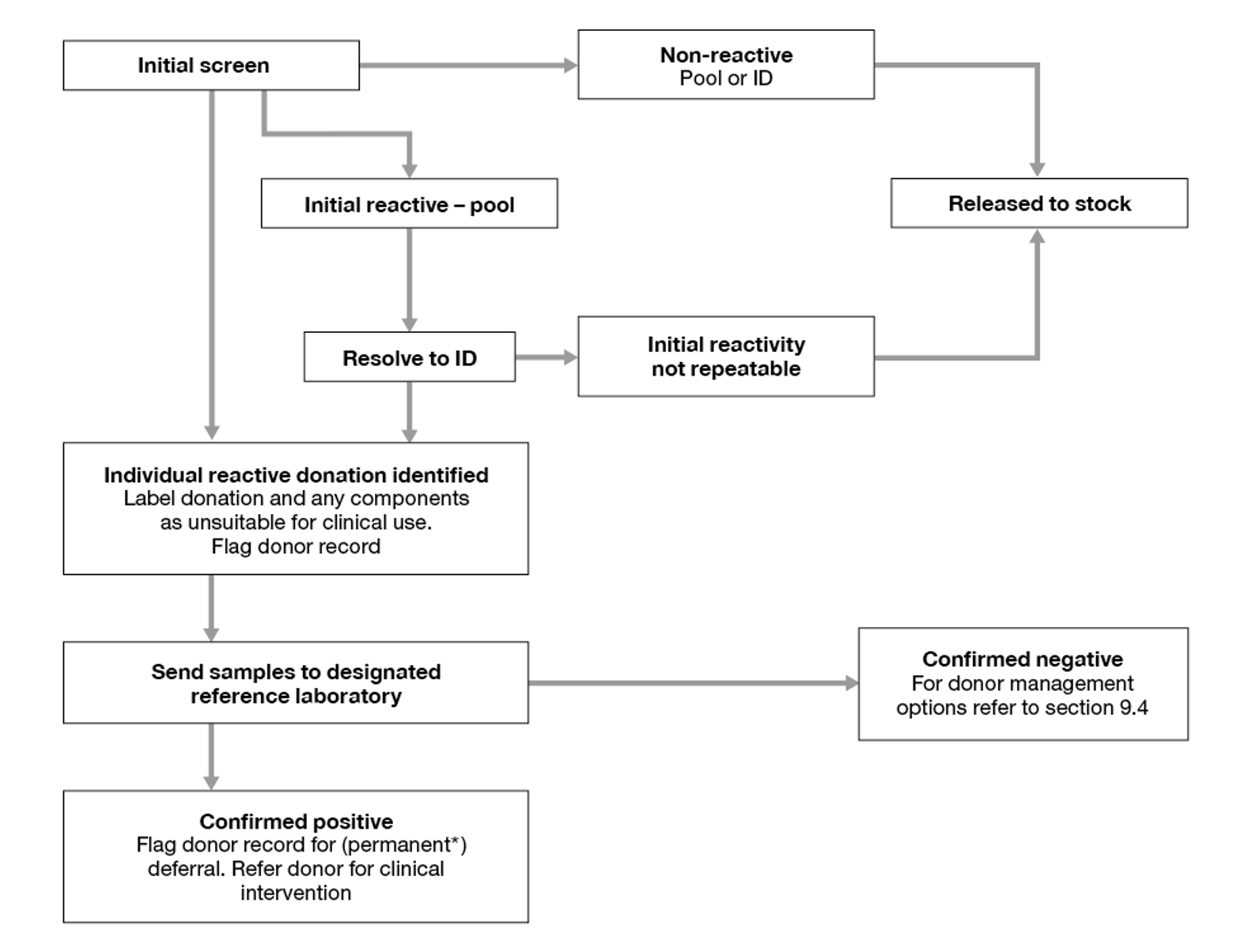
Figure 9.3 Molecular screening: blood donations
* Donors confirmed to be HEV or WNV RNA positive need only be deferred for 6 months from initial detection.
9.2.5: Confirmatory testing
When a donation is screen reactive for any of the serological or molecular mandatory or additional microbiology tests described above (except for anti-HCMV) samples from the donor/donation must undergo confirmatory testing at a designated reference laboratory.
- For blood, donations that are confirmed positive for anti-HBc from donors with anti-HBs >100 mIU/mL, tested in the past 24 months by a UK Blood Service, are considered suitable for release if HBsAg and ID HBV DNA negative.
- For tissues and cells either donations that are anti-HBc reactive and anti-HBs ≥100 mIU/mL are considered suitable for release, or donations which are anti-HBc reactive and are HBsAg and ID HBV DNA negative are considered suitable for release without the need for anti-HBs level of ≥100 mIU/mL.
- Anti-T. gondii IgG and IgM screening is recommended for HSC, donor lymphocyte infusions and other therapeutic cells (e.g. selected and cultured products including T-cells, natural killer cells, mesenchymal stem cells, cytotoxic T-lymphocytes, T-regulatory cells, tumour derived cells) and embryonic stem cell lines intended for clinical use derived from human embryos initially created for fertility treatment with the use of IgM positive donors avoided. Confirmation of anti-T.gondii IgM only reactivity recommended due to known specificity issues with IgM assays.
- If HEV, HAV or WNV RNA is confirmed in a donor, the donor record must be flagged as ‘temporary exclusion’ for 6 months. The donor can be reinstated automatically at least 6 months after the date of the index HEV, HAV or WNV RNA positive donation: see section 9.4.
- If human B19 DNA is confirmed in a donor, the donor record must be flagged as ‘temporary exclusion’ for 4 weeks. The donor can be reinstated automatically at least 4 weeks after the date of the index DNA positive donation: see section 9.4.
- In all other cases, the donor record must be flagged as ‘permanent exclusion risk – not to be used for clinical use’ or equivalent.
- In all cases where a positive result is confirmed, arrangements should be made to inform the donor and to ensure that the donor is given appropriate advice.
Note: Autologous stem cell donations may be collected from individuals who are known to be infected with one or more of the infectious agents for which donations are routinely screened. Such individuals are not generally classified as donors for the purposes of these guidelines.
- If a negative, inconclusive or indeterminate result is reported following confirmatory testing, and the initial reactivity is determined by the reference laboratory to be non-specific, use of further donations or the same donation (tissue and stem cell donors only) may be possible, as covered in section 9.4.
9.2.5.1: Specific requirements for HBsAg confirmation
The designated reference laboratory should, when appropriate, perform specific neutralisation tests for HBsAg to ensure that donors with low-level HBsAg reactivity, in the absence of other HBV markers, are not incorrectly reported as non-specifically reactive.
9.2.6: Definitions
| Term | Definition |
|---|---|
| Non-reactive (NR) | A sample whose reactivity when first tested falls below the assay cut-off as defined by the manufacturer’s instructions. May also be referred to as a ‘Negative’ test result |
| Initial reactive (IR) | Any sample whose reactivity when first tested falls above the cut-off as defined by the manufacturer’s instructions |
| Repeat reactive (RR) | Any sample reactive on two or more occasions either in the same screening assay (duplicate) or in two or more screening assays that are used in combination sequentially, to determine the suitability of a donation for release for clinical use |
| Alternative assay screening | When a second assay for the same screening target and of similar sensitivity is used sequentially to screen a sample which is either IR or RR in a first screening assay |
| Confirmatory testing | Full investigation, in a designated reference laboratory of a repeat reactive sample to determine whether the reactivity is specific to the infectious agent being screened for and indicative of current or past infection in the donor |
| Positive | A sample whose reactivity in confirmatory testing meets pre-defined criteria. This may indicate current or past infection |
| Inconclusive | A sample whose reactivity in confirmatory testing is not sufficient and/or specific enough to determine whether it reflects infection or possible non-specific reactivity |
| Negative | A sample whose screen reactivity, on investigation, is either not demonstrable or is deemed not to reflect infection |
| Individual nucleic acid test (ID-NAT) | Molecular screening of a donation as an individual sample cf. pooled testing |